Manipulating Water
Today, we explored some crazy ways to change how water works!
Particularly, we manipulated the pressure of water and water vapour within a closed system! Here’s what we found from the experiment:
1) We can change the pressure exerted by fluids within a closed system by changing their temperature. Particle theory states that as the temperature decreases, the kinetic energy of the particles decreases and the particles get closer together and decrease pressure. Likewise, as the temperature increases, kinetic energy increases and particles spread out and increase pressure.
By cooling the gas at the top of the beaker, we are able to lower the temperature and the pressure exerted by the gas on the liquid (water).
2) As the pressure exerted by the gas is reduced within the closed system, it makes it easier for particles of the liquid to break free and vaporize. In essence, this is because there is less force pushing down on the liquid particles to prevent them from escaping.
3) The freezing point and boiling point of substances depends on the pressure. At normal atmospheric pressure (atm), the freezing point and boiling point of water are at 0 ℃ and 100 ℃ respectively. In particular, when the pressure decreases, the boiling point of water also decreases. This is why by cooling the water vapour within the beaker, the previously heated water is able to vapourize and boil.
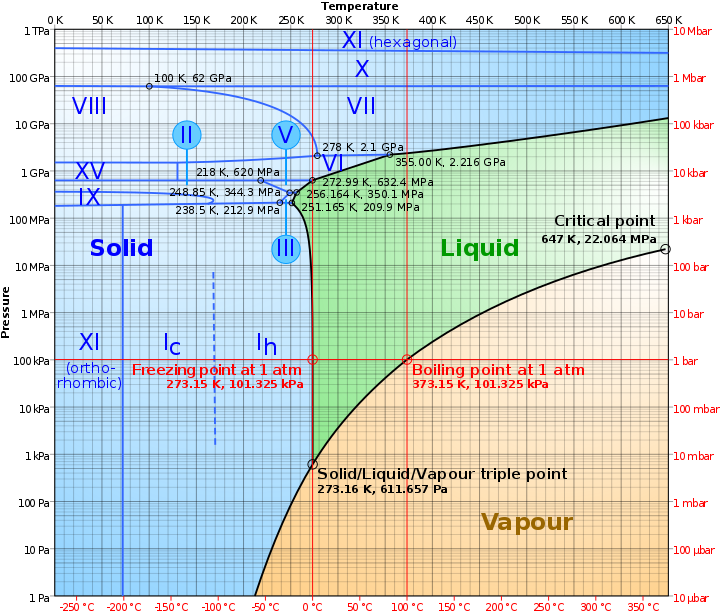 Graph of how the state of water is determined based on varying temperature and pressure!
Graph of how the state of water is determined based on varying temperature and pressure!
Here are a few questions that I have:
1) What happens when the pressure approaches zero (i.e. a vacuum)? If we put water in a closed system and suck out all the gas to eliminate the pressure, will it freeze?
2) How does supercooling and superheating relate to these concepts? Do they depend on pressure as well?
An intriguing idea that I discovered today was the triple point of water! Under certain pressure conditions, water at 0.01 ℃ can exist in all three states: solid (ice), liquid (water), and gas (water vapour)! The problem with achieving this feat is that it’s very hard to achieve the specific pressure and temperature required due to many limitations and natural tendencies in physics.