
Latent Heat
Today, we explored the final part of heat: latent heat!
Just like with specific heat, we conducted an experiment with a metal plate and a plastic plate, but this time we melted ice on both of the plates! I have to say, it was almost just as exciting as watching paint dry. The experiment really helped show how latent heat worked, and here’s what I learned:
1) Thermal energy is made up of the internal kinetic energy of matter as well as latent heat, which comes from the energy released or absorbed when matter changes state. Liquids can freeze to release energy, while gases can condensate to release energy and then vapourize to absorb energy.
2) As latent heat is energy and this energy must be transferred in order to change a state, when an object reaches the fusion (freezing or melting) point or the vaporization (boiling or condensation) point the energy that goes into changing the temperature temporarily goes to changing the state of the object, stopping the temperature of the object from changing. In the case of water, the graph of the temperature of ice (water in solid state) melting into water (in liquid state) would look like this:
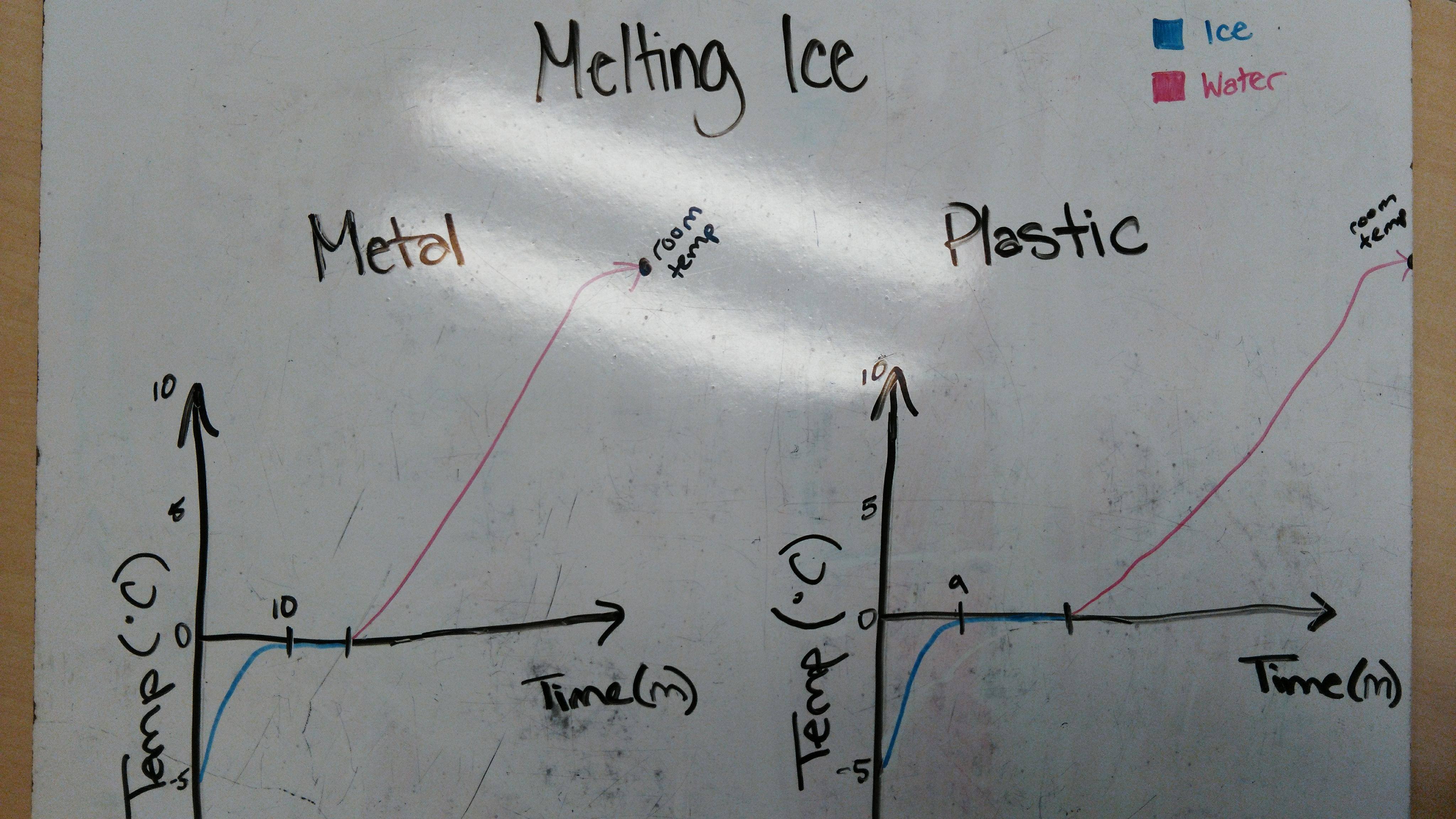 Notice how the slope of the graph goes to zero at the freezing point of water! Latent heat is stopping the external energy from raising the temperature.
Notice how the slope of the graph goes to zero at the freezing point of water! Latent heat is stopping the external energy from raising the temperature.
3) Each object has their own specific latent heat, which helps us find the internal potential energy of an object. Since specific latent heat describes the ratio of potential energy to a kilogram of matter, it is expressed in terms of J kg-1. Then, to find the potential energy, we would just multiply the specific latent heat by the amount of matter, the mass:
Thermal energy (J) = Mass (kg) * Specific Latent Heat (J kg-1)
Q = mL
Here are my questions on the topic of latent heat:
1) Does a solid have any internal potential energy? Is it possible for it to release energy and transform into an even lower state of matter? Likewise, does latent heat still apply to states of matter with higher temperature, like plasma?
2) There is a way for matter to transform from its gaseous state into its solid state, which is called sublimation. How does latent heat work in this scenario?
An intriguing idea that I discovered today was that while some materials may have a higher specific heat capacity than others, what matters most in being faster in changing the thermal energy of an object depends more on your power rather than your potential energy. This is why the metal disk was able to melt the ice faster, despite the plastic having slightly more specific heat capacity.
After Unit Reflection
Understanding Energy - Thermal Energy
In this blog post and my previous blog post on specific heat, I gained an understanding of the two forms of energy that make up thermal energy: internal kinetic energy and latent heat. I made a key observation that thermal energy, the transfer of energy from a hotter body to a colder body occurs because of world’s natural tendency towards increasing entropy, or disorder (2nd law of thermodynamics).
I expressed my knowledge about the internal kinetic energy of matter from showing that it was given by the temperature, mass, and specific heat with the equation Q = mcΔT. A higher specific heat meant that it took more energy to change the temperature of an object, which explained why putting water in a balloon made it harder to pop.
In addition, I showed how latent heat came from the energy that can be released or absorbed when matter changes state, and was represented by the equation Q = mL. Latent heat needed to be overcome before changing state, and was the reason why no temperature changes occurred for a while at the freezing and boiling points of a substance.
As for both specific heat and specific latent heat, I noted that both values were specific to different materials and needed to be documented as a dictionary.